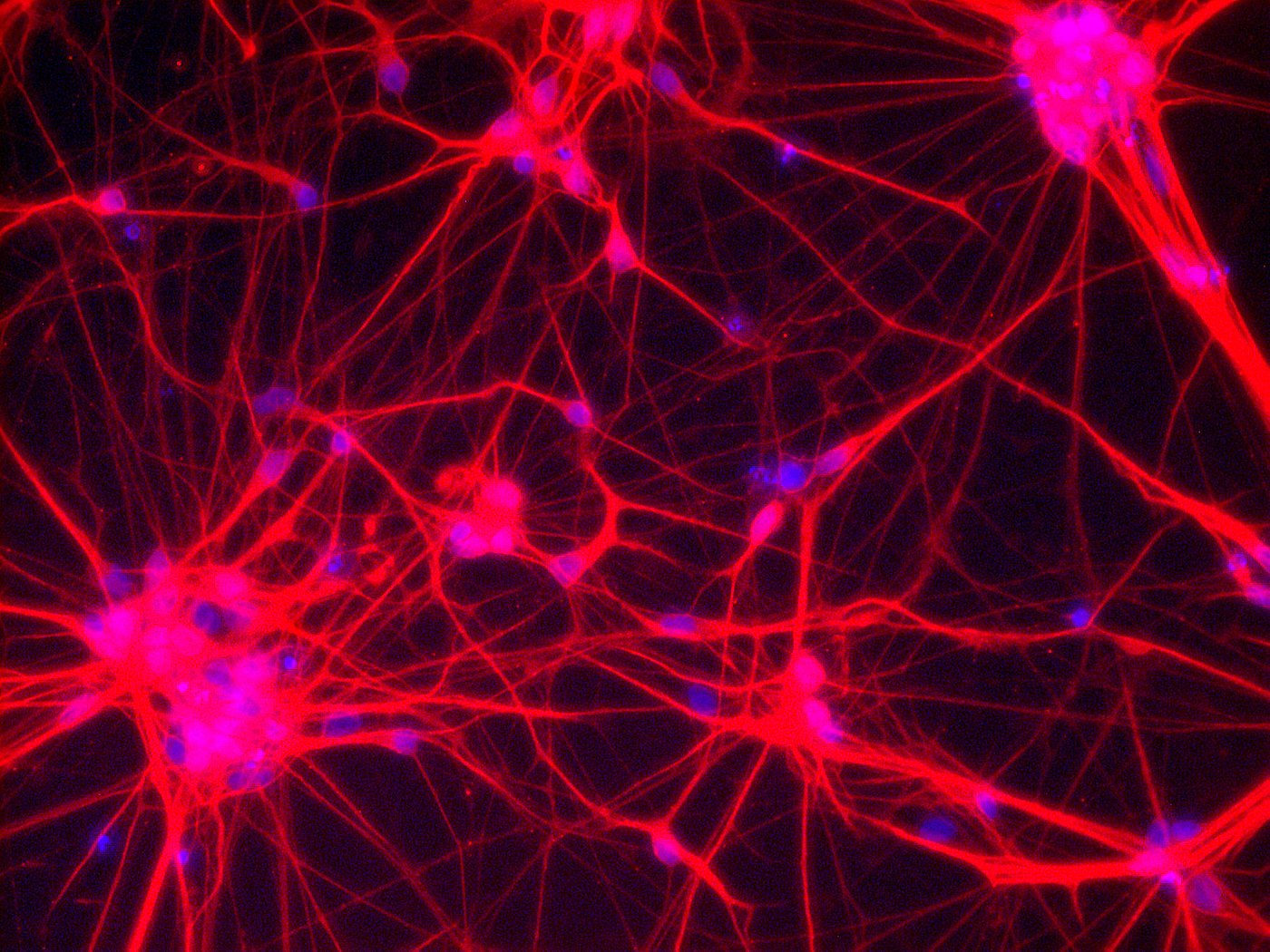
Neurons – Network of neuronal cells (Blue: nucleus; Red: neurons) [Copyright: Institute for Transfusion Medicine, UK Essen]
At present, there are only a few approved stem cell-based therapies; numerous new approaches are still being tested in clinical trials. However, patients are also confronted with stem cell-based treatment options that are not being clinically researched or that have not (yet) been approved. As is common for healthcare interventions, providers are obliged to inform patients about relevant facts beforehand. This is to ensure that patients can give "informed consent" to the procedure. With the help of experts from clinical practice, ethics, research and regulation, the ISSCR has listed what information should be given regarding stem cell-based treatments performed outside of clinical trials. Information is required, for instance, on potential risks, scientific support for anticipated benefits, expertise of the team administering the intervention, existing approvals, and (follow-up) costs. Although the document is aimed primarily at healthcare professionals, it can also point patients to information they should demand before choosing treatment options. Patients should thoroughly evaluate interventions performed outside clinical trials against these criteria.
Available for download free of charge: Informed Consent Standard for Stem Cell-Based Interventions Outside Clinical Trials
Further patient information on stem cell-based treatments can be found here.