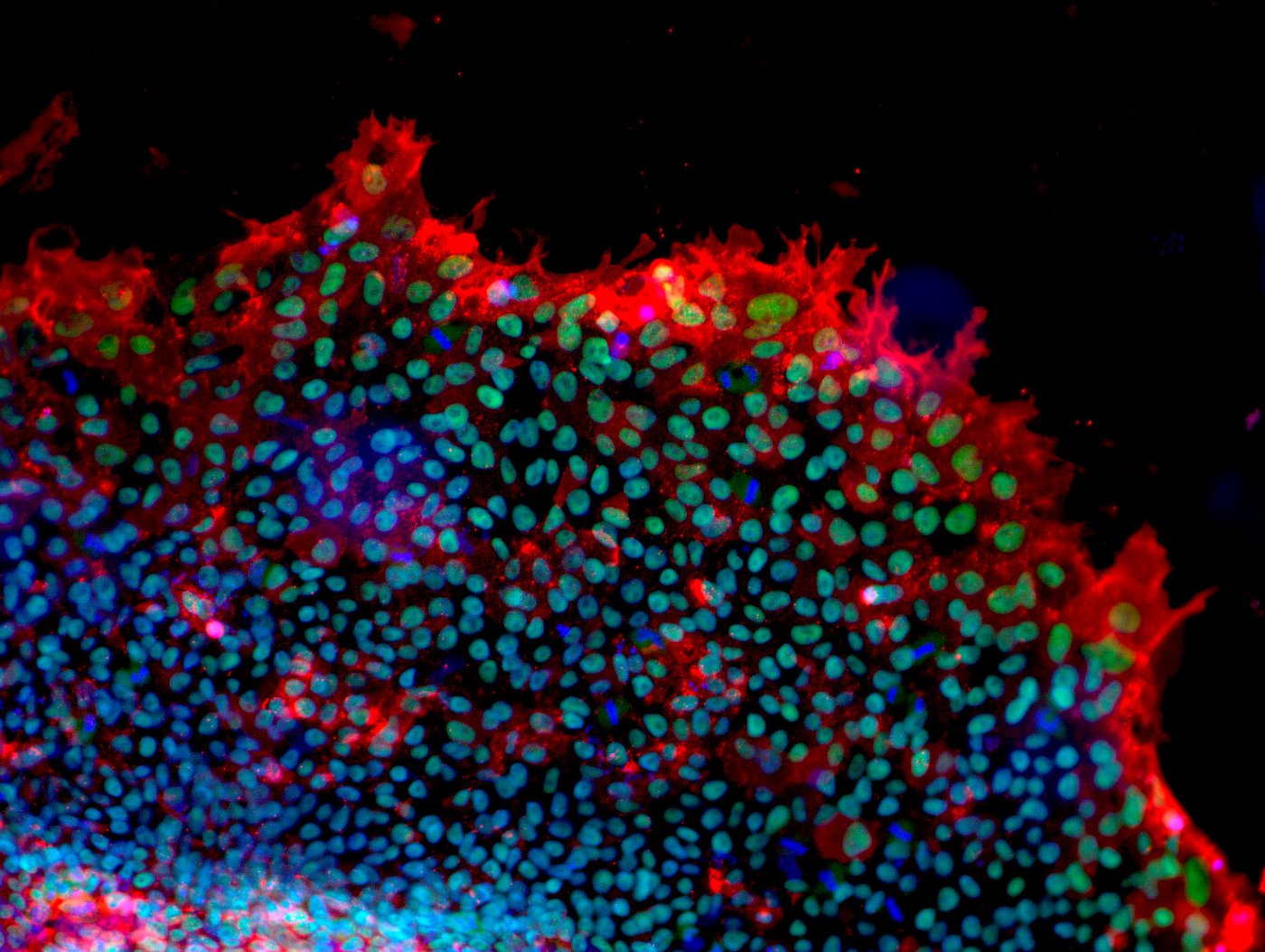
Human mesenchymal stromal cells from adipose tissue - The cells were differentiated into fat cells and fat droplets were stained green. Cell nuclei were stained blue. [Copyright: Institute for Biomedical Engineering – Cell Biology, UK Aachen]
Press releases and news about progress in stem cell research are published nearly every day. This in turn causes that there are high expectations in the population for the cure of severe diseases with stem cells. Especially patients with so far incurable diseases hope for a stem cell-based treatment or therapy. However, the assessment of new research results, especially with regard to whether and when they could be available for patients, is very complex.
There are only a very limited number of diseases for which stem cell-based treatments have been approved. Most stem cell-based approaches are still in preclinical or clinical research and are not licensed yet to be applied to the patient.
The Stem Cell Network North Rhine-Westphalia wants to inform about currently approved stem cell-based treatment options and to provide the appropriate questions and resources you may need to make the best decision about a possible treatment.
Therefore, we have selected various materials:
Various private clinics around the world offer stem cell-based therapies, especially on the internet. Often, treatments are offered with the same procedure for a plethora of diseases. Various media reported about it and the recently an important judicial ruling took place to stop questionable stem-cell clinics. Articles are listed chronologically:
„Stem cells have been called everything from cure-alls to miracle treatments. But don’t believe the hype. Some unscrupulous providers offer stem cell products that are both unapproved and unproven. So beware of potentially dangerous procedures—and confirm what’s really being offered before you consider any treatment." (Read the full warning of the U.S. Food & Drug Administration)
The European Medicines Agency (EMA) is “advising patients and the general public against using unregulated cell-based therapies which may not be safe or effective. […] Patients or their families who are considering cell-based therapies should ask their healthcare professional about the benefits and risks of the treatment and which authority has approved it.” (Read the full warning of EMA against using unproven cell-based therapies)