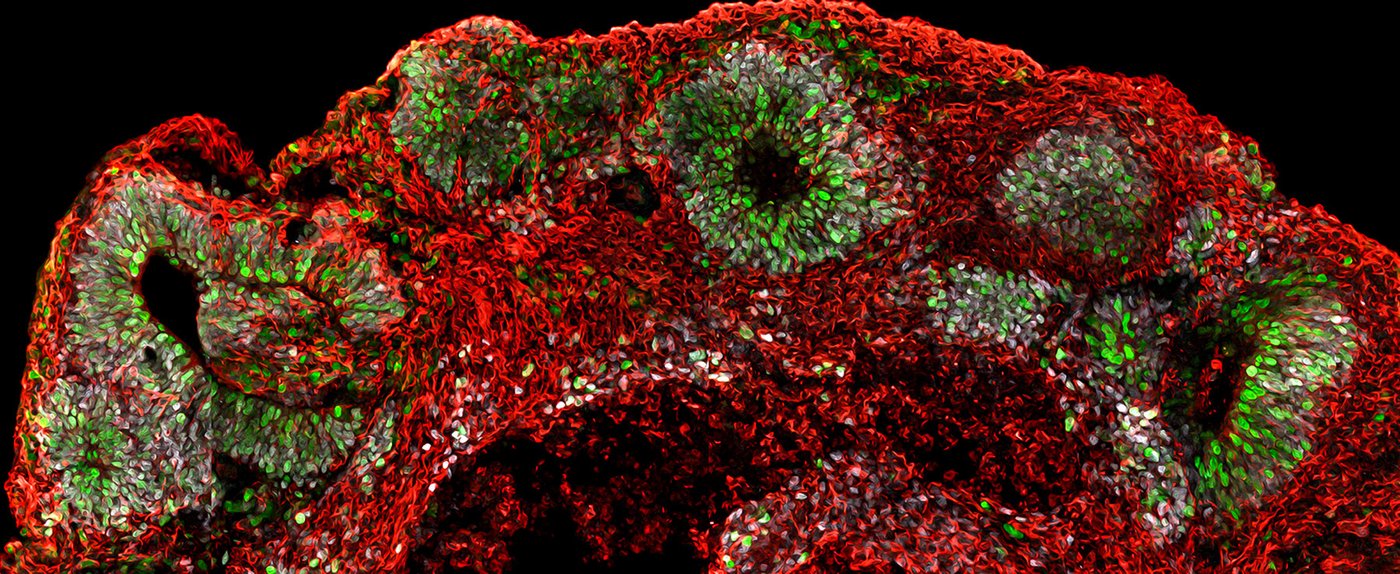
Human brain tissue in a dish: Young cerebral organoid generated from human iPSCs (Green: proliferative cells, White: neural progenitor cells, Red: neurons) [Copyright: Max Planck Institute for Molecular Biomedicine, Muenster]
The aim of clinical studies is to test the safety and efficacy of medicaments, forms of therapy or medical devices. Clinical studies are composed of different phases and pursue both the investigation of scientific questions as well as the improvement of medical treatment options. They are an important tool to translate promising research results from the laboratory to the safe application in patients.
Centers for clinical studies are usually central facilities of university hospitals that support scientists to conduct clinical studies. Comprehensive information on centers for clinical studies can be found (in German) on the website of the „KKS-Netzwerk e.V.“, which is an joint association of the coordination centers for clinical trials in Germany (KKS) and the centers for clinical trials in Germany (ZKS) that aims to strengthen patient-oriented clinical research in Germany. Furthermore there is the European Network ECRIN, which aims to facilitate European clinical research.
Extensive information on how clinical studies are conducted can be found on the internet pages of the Federal Ministry of Education and Research: https://www.gesundheitsforschung-bmbf.de/de/wie-funktionieren-klinische-studien-6877.php
The information is only available in German.
Thanks to the high density of university hospitals in North Rhine-Westphalia there are more centers for clinical studies than in other German federal states. These are listed alphabetically below: