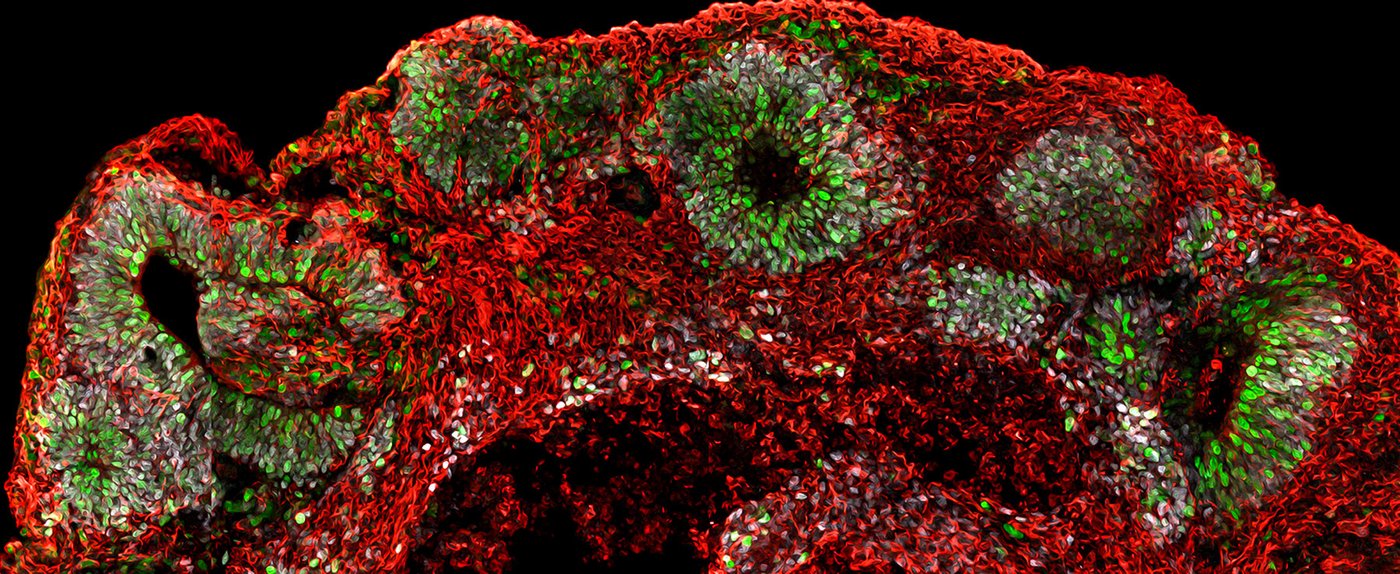
Human brain tissue in a dish: Young cerebral organoid generated from human iPSCs (Green: proliferative cells, White: neural progenitor cells, Red: neurons) [Copyright: Max Planck Institute for Molecular Biomedicine, Muenster]
The term translation refers to the transfer of new research findings from the laboratory into clinical application. Basic research forms the basis for this process. Promising research approaches are then tested first in preclinical studies and then on patients in clinical phases.
In recent years, new findings and technological advances within stem cell research have led to numerous clinical trials using stem cells worldwide. The aim here is to use stem cells to develop new therapies or drugs. Adult stem cells have predominantly been used up to now, but there are also promising approaches using embryonic and induced pluripotent stem cells.
The transfer of findings from basic research into use on patients always presents scientists with new challenges. For example, findings from (pre-)clinical studies must be fed back into basic research in the laboratory, quality standards must be maintained, and regulatory challenges in the approval of clinical therapies must be overcome. In addition, it is very important that research institutions, hospitals, and, ultimately, industry are in close and continuous communication with each other.
The Stem Cell Network North Rhine-Westphalia would like to support researchers in North Rhine-Westphalia (NRW) along this path in the best possible way. Scientific, ethical, legal, social scientific, and regulatory obstacles all have to be overcome as part of this. This will make it possible, for example, for new stem cell-based therapies and drugs to be approved, thus allowing the potential of stem cell research to be used efficiently.