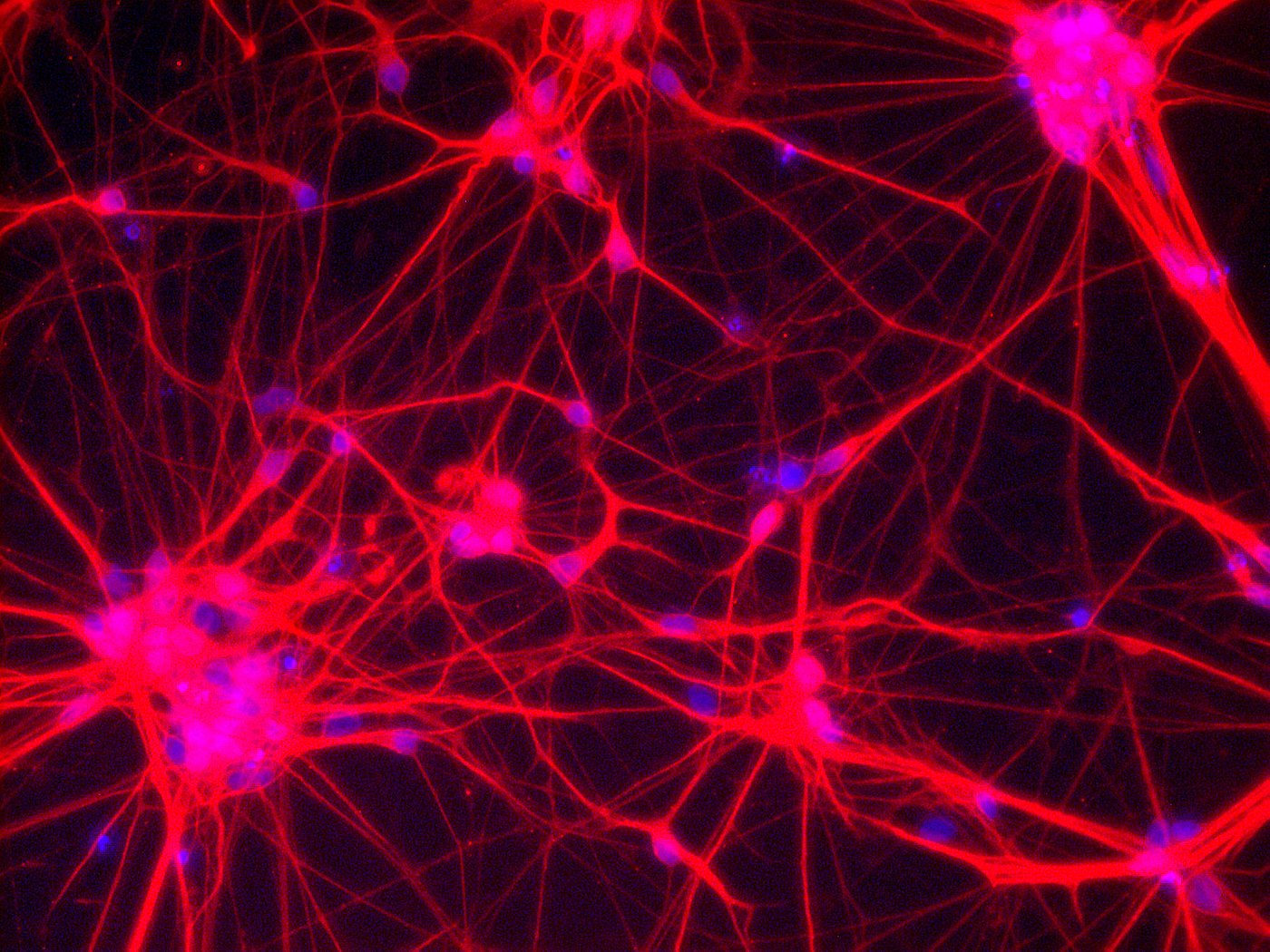
Neurons – Network of neuronal cells (Blue: nucleus; Red: neurons) [Copyright: Institute for Transfusion Medicine, UK Essen]
In recent years, stem cell research has developed at a rapid pace. In addition to basic research, disease models and drug testing technologies, therapeutic approaches based on stem cells are also increasingly approaching realization. With several hundred clinical studies on stem-cell-related therapeutic treatments, the importance of the field for clinical translation is clearly also increasing. At the same time, in the interests of the patients as well as of the research field as a whole, the therapeutic use of stem cells requires a thorough and rigorous examination of the procedures. It is therefore crucial that clinical trials have sound research design and are based on adequate preclinical data.
On the basis of interdisciplinary expertise, the ISSCR has set itself the task of contributing to public awareness on stem cells and the opportunities they provide, and not least also on the risks. In addition to a variety of stem cell-related information material (see http://www.closerlookatstemcells.org/), a patient guide has also been developed to help potential patients make a (scientifically) informed decision about possible stem cell treatments, and is now available in ten languages.
The newly published guideline “Stem Cell Based Clinical Trials” aims to provide supervisory and regulatory authorities with a sound decision-making basis for clinical trials. The field is now so large and diverse that even well-informed decision makers have difficulty keeping track. At the same time, regulations and responsibilities are internationally very heterogeneous. While government regulators in many cases have well-standardized evaluation procedures, this is not always ensured for local clinical ethics committees. With the guideline, the ISSCR wants to support decision-making authorities in order to reduce the risk to the patient's well-being and to prevent harm to the research field as a whole.
In order to enable a broad application of the guideline, independent of stem cell source and treatment design, the guideline does not aim to impart the (constantly evolving) state of research. Instead, the user is given key questions for various areas. In the categories: (1) general, (2) cell product, (3) preclinical data, and (4) study design, a total of 29 questions are listed that should be answered for the adequate assessment of a study. Additionally, there is a category of signs that may indicate potentially problematic clinical trials. The guideline is available for download here.