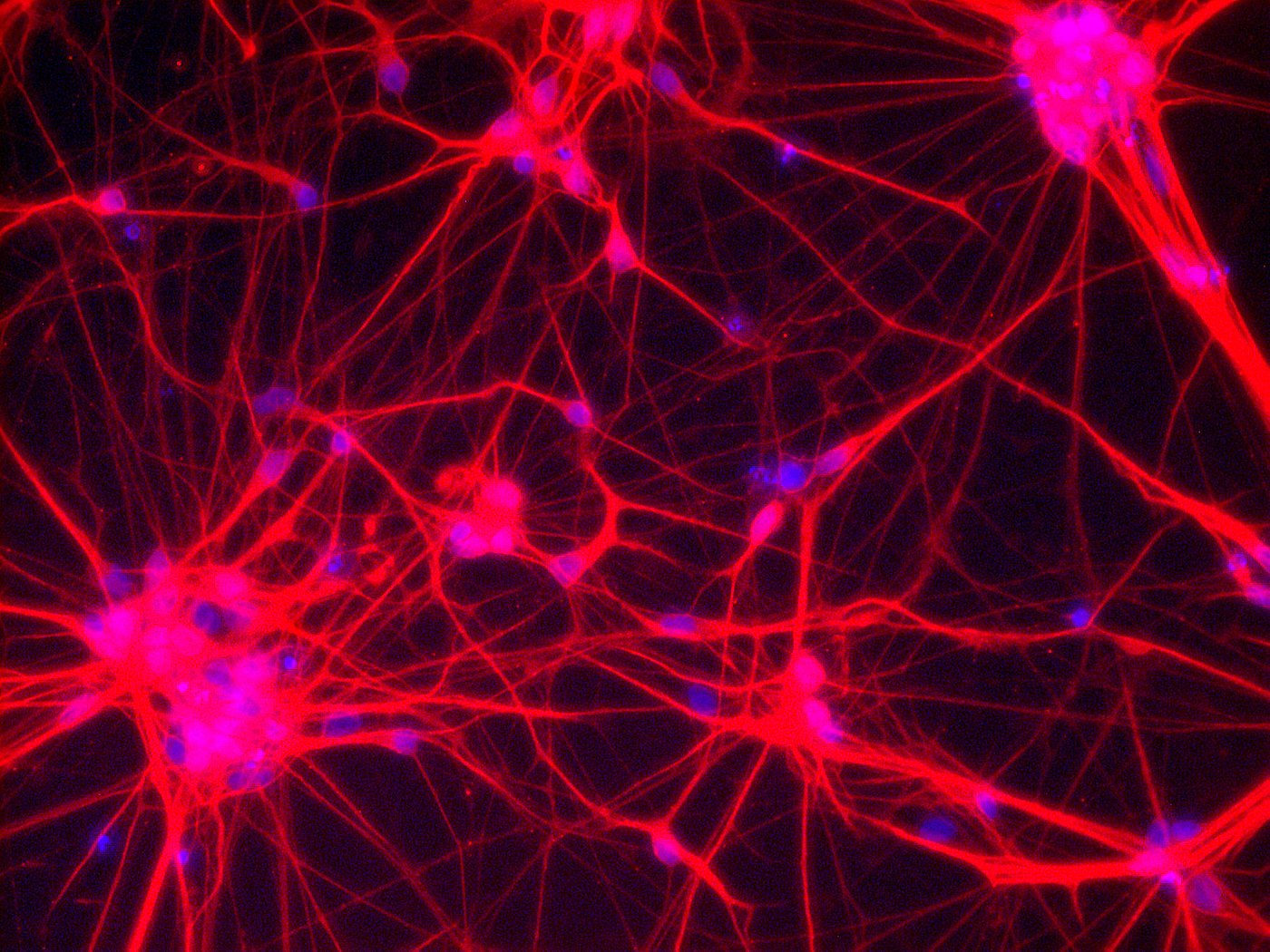
Neurons – Network of neuronal cells (Blue: nucleus; Red: neurons) [Copyright: Institute for Transfusion Medicine, UK Essen]
In recent years, stem cell research has developed at a rapid pace. With several hundred clinical studies on stem-cell-related therapeutic treatments, the importance of clinical translation is clearly also increasing. It is therefore crucial that clinical trials have sound research design.
The recently published guidelines “Stem Cell Based Clinical Trials” aim to provide supervisory and regulatory authorities with a sound decision-making basis for clinical trials. Regulations and responsibilities are internationally very heterogeneous and, furthermore, the field has become quite large and diverse, so that even well-informed decision makers have difficulty keeping track. With the guidelines, the ISSCR wants to support decision-making authorities in reducing the risk to patients’ well-being and to prevent harm to the research field.
Besides these new guidelines the ISSCR has already published a variety of stem cell-related information material (see http://www.closerlookatstemcells.org/). For example, a patient guide has been developed to help potential patients make a (scientifically) informed decision about possible stem cell treatment, and is now available in ten languages.
Available for download:
Original version: “Stem Cell Based Clinical Trials – Practical Advice for Physicians and Ethics/Institutional Review Boards”
German translation: “Stammzellbasierte klinische Studien – Praktische Hinweise für Ärzte und Ethikkommissionen / Institutionelle Prüfungsgremien“