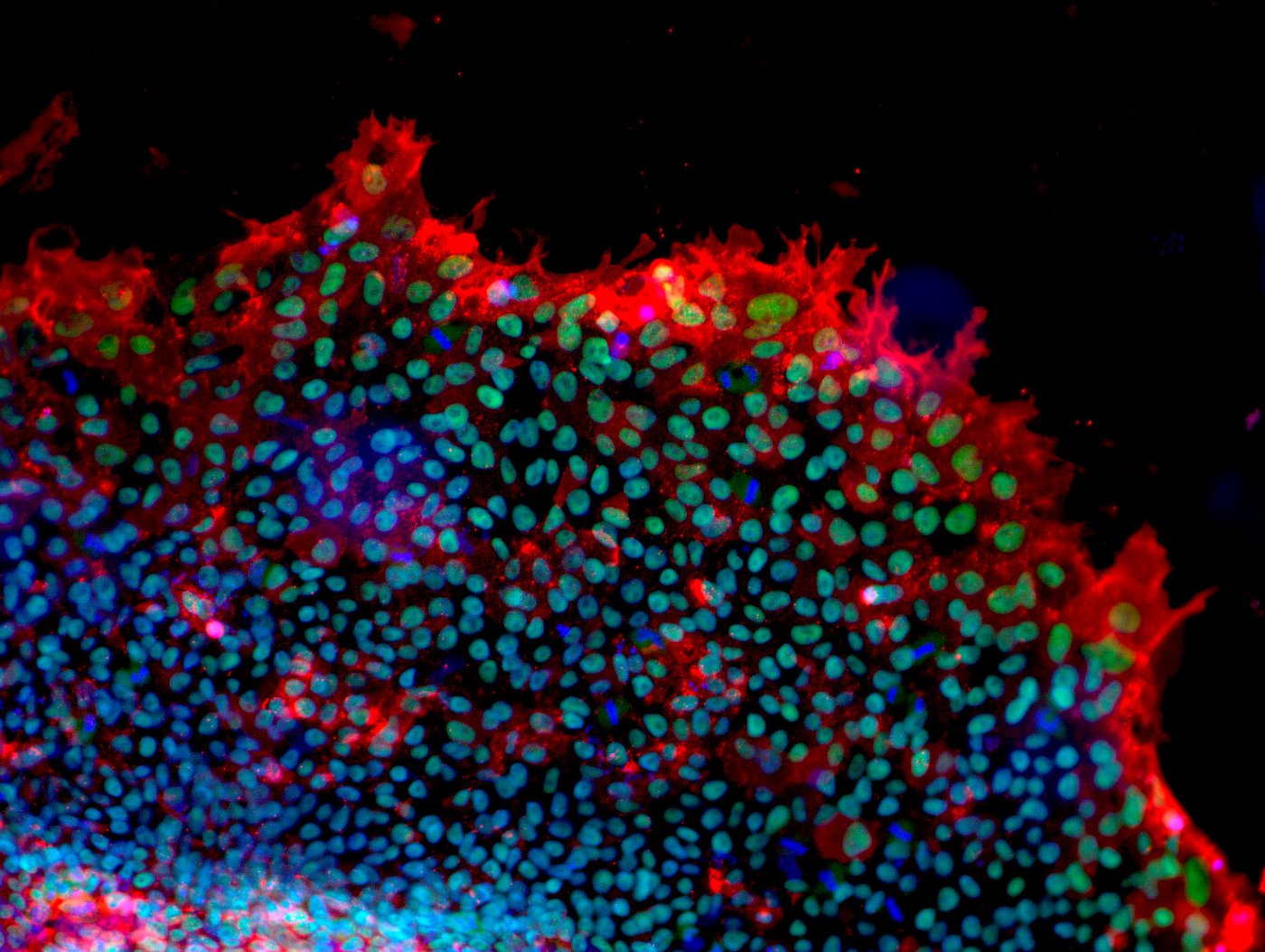
Human mesenchymal stromal cells from adipose tissue - The cells were differentiated into fat cells and fat droplets were stained green. Cell nuclei were stained blue. [Copyright: Institute for Biomedical Engineering – Cell Biology, UK Aachen]
Embryonic stem cells (ES cells) are not found in the adult body. They only occur at a very early stage of embryonic development, the blastocyst. This is a developmental stage that a human embryo goes through on the 5thday after fertilization. At this early stage, no organs have been formed, it is merely a kind of cell cluster (see Figure 2).
In order to obtain embryonic stem cells, the outer cell layer of the blastocyst must be destroyed so that the remaining cells of the inner cell mass (embryoblast) can be isolated and converted into cell cultures in the laboratory (see Figure 3). This internal cell mass, i.e. the isolated cells, has the potential to develop under controlled conditions into any cell type of the human body, but the embryonic stem cells can no longer form a complete organism.
This characteristic is called pluripotency (Latin: many abilities); in contrast to the totipotent, single-cell embryos (=originating cell), which are always at the very beginning of individual development. Pluripotent stem cells are characterized by the ability to produce all three cotyledons (ectoderm, endoderm, mesoderm). They are, therefore, not yet fixed to a specific tissue type (see Figure 4).
Research is carried out worldwide with both animal and human embryonic stem cells (hES cells). In the laboratory, it is possible to produce certain cell types, e.g. nerve cells, from embryonic stem cells by adding certain growth factors. This is called differentiation (see Figure 4). In addition, the cells can be multiplied in the laboratory and kept in cell culture. In order to produce hES cells, blastocysts are usually used that have been produced for artificial insemination (in vitro fertilization) but are no longer used for implantation. In Germany, the production of hES cells in the laboratory is prohibited. However, it is possible to import hES cells from other countries for research purposes under strict conditions.
Another way of producing embryonic stem cells is what is known as therapeutic cloning, which is based on somatic cell nuclear transfer (SCNT). In the case of a donated egg cell, the cell nucleus is removed and the nucleus of a somatic cell is used instead. The cells obtained by this method are also pluripotent. The method has so far only been used on animal cells. Further information on cell nuclear transfer can be found on the website of the German Reference Centre for Ethics in the Life Sciences (DRZE).
Figure 2 and 3 were kindly provided by the German Reference Centre for Ethics in the Life Sciences (DRZE).
Adult stem cells (also known as somatic or tissue stem cells) are already specialized stem cells that can only form certain cell types of the human body. For example, skin stem cells can only produce different cell types of the skin, but not blood or nerve cells. This ability is known as multipotency (Latin: more than one ability). Multipotent stem cells can develop into different cell types for a certaintissue, i.e. they are usually only able to form cells of a certain cotyledon (cf. Fig. 4). It is now assumed that adult stem cells occur in all tissues of the human body and can regenerate it, for example in the case of injury. If, for instance, the skin is injured by a cut or burn, the skin stem cells there are activated and form new skin cells through division and differentiation.
In recent years, science has discovered new types of adult stem cells in tissues in which no stem cells had previously been suspected. For example, while it was previously assumed that the tissues of the human eye could not regenerate, it is now known that there are stem cell depots in the cornea of the human eye. The best-known type of adult stem cells arethose of the hematopoietic system found in the bone marrow. They have already been used for 50 years to successfully treat diseases of the hematopoietic system (such as leukemia).
Mesenchymal stem cells (MSCs), which are also found in bone marrow, are particularly noteworthy among adult stem cells. MSCs offer great potential for many therapeutic applications and are already being investigated in numerous clinical studies.
In 2012, the Nobel Prize for Medicine was awarded to the Japanese stem cell researcher Shin’ya Yamanaka and his British colleague Sir John Gurdon. Based on a Gurdon method, Yamanaka succeeded in rejuvenating (reprogramming) mature cells of an adult organism by using transcription factors, i.e. returning the development of the cells to an early embryonic state. These cells were then almost as versatile as embryonic stem cells and could form many different tissue types, e.g. nerve cells or blood cells (see Figure 5). They are thus also pluripotent, as are embryonic stem cells.
Reprogramming was initially only possible with animal cells. In 2007, various research groups also succeeded in reprogramming human cells. Since then, the reprogramming techniques, which were initially very inefficient and potentially risky, have developed considerably.
Nowadays, cell lines exist that do not have the transcription factors permanently in the genetic material and provide a good basis for scientific questions. Whether iPS cells actually fully correspond to ES cells in their abilities is a question that has not yet been clarified. iPS cells have the advantage over ES cells that no embryos have to be used to produce them. In addition, the reprogrammed cells are compatible with the immune system of the cell donor and are therefore particularly promising for therapeutic purposes.
There are other stem cell types in addition to adult and embryonic stem cells. In particular, the umbilical cord blood stem cells, which are contained in the blood of the umbilical cord or placenta after cutting the cord, should be mentioned here. According to current knowledge, umbilical cord blood stem cells are not pluripotent. However, their potential is higher than that of other, multipotent stem cells.
In contrast to (adult) stem cells, the specialized progenitor cellsof the adult organism can only ever produce further cells of a specific cell type; for example, no liver cell or hair cell can develop from a blood progenitor cell during division. This characteristic is called unipotency (Latin: one ability).
Further information on the definition of stem cells, their properties and categories, and the state of research can be found here.
A certain cell type – such as a neuron – can be produced from an already differentiated somatic cell – e.g. a skin cell – by first generating a pluripotent stem cell from the initial cell (dedifferentiation) in order to then generate the specialized “desired cell type” from it, e.g. a neuron (redifferentiation; cf. Figure 5). In the meantime, however, scientists are also able to reprogram a specialized cell “A” directly into another specialized cell “B”, i.e. without the detour via a pluripotent intermediate. This process is called transdifferentiation. This works, similar to the differentiation processes described above, by bringing the cells into contact with cell type-specifictranscription and growth factors (see Figure 6). Since no detour via iPS cells is necessary, transdifferentiation can generate the desired specialized cells faster than with other methods. Moreover, no embryonic tissue is required. One disadvantage, however, is that the cells retain at least part of their cellular “memory” (e.g. epigenetic markers) and are therefore not completely comparable with their naturally occurring “copy”.